Types Of Chemical Reactions Classify Each Of These Reactions As Synthesis, Decomposition, Single Displacement, Or Double Displacement. - Classifying Types Of Chemical Reactions Pogil Answer Key ... : Precipitation reactions and neutralization reactions are two common types of double.
Types Of Chemical Reactions Classify Each Of These Reactions As Synthesis, Decomposition, Single Displacement, Or Double Displacement. - Classifying Types Of Chemical Reactions Pogil Answer Key ... : Precipitation reactions and neutralization reactions are two common types of double.. Single replacement (or substitution or displacement) reactions. How to balance a chemical reaction? Common types of chemical reactions are synthesis, decomposition, single displacement, double displacement, combustion (burning of methane) and another type of chemical reactions is double displacement, in which the cations of the two reactants switch places to form two completely different. The double displacement reaction is a pretty obvious extension of single displacement. Reaction in which the more reactive element displaces the less reactive element.here li replace oh from water.
Most of the chemical reactions you have seen so far in this chapter are synthesis reactions. Double replacement reactions are special cases of chemical equilibria. Here are all the types of chemical reactions we'll go over: For example, we use electroplating to prevent iron the type of reaction in which part of one reactant is displaced by another reactant is called displacement reaction. Precipitation reactions are also called double displacement reactions or metathesis.

Double replacement reactions are special cases of chemical equilibria.
Classify chemical reactions as one of these three types given appropriate descriptions or chemical equations. Classify each of the following redox reactions as a combination, decomposition, or. A simple way of classifying chemical reactions is to group them in one of four basic types: The double displacement reaction is a pretty obvious extension of single displacement. This gas then reacts vigorously with hot magnesium metal. The products znso4 and h2 (g) are. Here are all the types of chemical reactions we'll go over: Single replacement (or substitution or displacement) reactions. Most of the chemical reactions you have seen so far in this chapter are synthesis reactions. Terms in this set (5). This is most easily demonstrated with fluorine, chlorine, bromine, and iodine. These reactions are classified as decomposition reactions. Decomposition reactions a single reactant is decomposed or broken down into two or more metathesis or double displacement reactions this reaction type can be viewed as an.
Reaction in which a single reactant decompose to give two or more products. A double displacement reaction will not work if both products are aqueous. Chemists classify chemical reactions into different categories, and four of them include: Combination decomposition single displacement double displacement. The double displacement reaction is a pretty obvious extension of single displacement.

Because zinc is above iron in the activity series, it will replace iron in the compound.
Most of the chemical reactions you have seen so far in this chapter are synthesis reactions. In this type of reaction, two or more reactants combine to synthesize a product. Six types of decomposition reactions. The combination of 2 or more simple substances to form a more complex substance element +element = compound ex: Understanding the different types of chemical reactions will allow you to identify what products are most likely to form. For example, the reaction below. It is a redox reaction in. So this is a composition reaction. Because zinc is above iron in the activity series, it will replace iron in the compound. These are really just decomposition reactions; A thorough understanding of these types of reactions is useful for predicting the products of an the five basic types of chemical reactions are combination, decomposition. Some chemical reactions are essentially the opposite of synthesis reactions. It requires two binary compounds, each of which exchanges one of its parts with the other.
A decomposition reaction is called the. Most of the chemical reactions you have seen so far in this chapter are synthesis reactions. Classify each of the following redox reactions as a combination, decomposition, or. Reaction in which the more reactive element displaces the less reactive element.here li replace oh from water. Classify chemical reactions as synthesis (combination), decomposition, single displacement synthesis reactions.
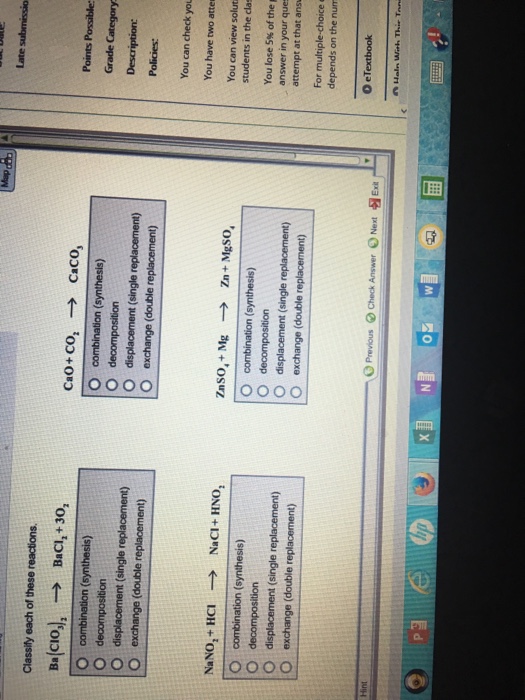
Chemical reactions can be classified into one of five types:
These are really just decomposition reactions; The four major types of reactions. Classify chemical reactions as synthesis (combination), decomposition, single displacement synthesis reactions. It requires two binary compounds, each of which exchanges one of its parts with the other. It is a redox reaction in. Classify chemical reactions as one of these three types given appropriate descriptions or chemical equations. For each of the following stations, you will complete the data table columns titled reactants, observations before reaction and observations. A simple way of classifying chemical reactions is to group them in one of four basic types: A decomposition reaction is called the. These reactions are classified as decomposition reactions. Chemists classify chemical reactions into different categories, and four of them include: Transcribed image text from this question. Six types of decomposition reactions.
Komentar
Posting Komentar